Bacterial biofilms are a considerable public health threat because they can cause chronic and hospital-acquired infections, as well as persistent biofouling of medical implants, but are resistant to antibiotics. Biofilm regulation by NO has been observed broadly in bacteria, but is poorly understood. For example, NO regulation of biofilm dispersal in Pseudomonas aeruginosa, a principal pathogen in cystic fibrosis and hospital-acquired infections, is well documented, but the primary NO sensor is unknown. Many bacteria use H-NOX domains as NO sensors, but P. aeruginosa does not encode one. This suggests the existence of a previously uncharacterized NO sensor.
We recently described the discovery of a novel nitric oxide binding protein (NosP; NO sensing protein), which is much more widely conserved in bacteria than H-NOX, as well as a novel NO-responsive pathway in P. aeruginosa (Hossain and Boon 2017). We have demonstrated that biofilms of a P. aeruginosa P. aeruginosamutant lacking components of the NosP pathway lose the ability to disperse in response to NO. Upon cloning, expressing, and purifying NosP, we have found it binds heme and ligates to NO with a dissociation rate constant that is comparable to other well-established NO-sensing proteins. Moreover, we have found that NO-bound NosP is able to regulate the phosphorelay activity of a hybrid histidine kinase (PA1976) that is involved in biofilm regulation in through an HptB-mediated phosphorelay pathway (figure 4). Thus, we have discovered a novel NO-responsive pathway that regulates biofilm in P. aeruginosa.
Discovery of the NosP family of sensing proteins has opened up many new and exciting lines of research in our laboratory. In P. aeruginosa, we are currently aiming to: (i) characterize the role of nosP in biofilm formation and dispersal, virulence, and antibiotic resistance; (ii) gain structural and functional insights into NosP by generating a high-resolution crystal structure as well as electronic and vibrational structures of the heme cofactor using spectroscopic analysis; and (iii) delineate the detailed signaling mechanism downstream of NosP. We are also investigating the role of NosP in other organisms to determine if its function in biofilm regulation is conserved as well as pursuing opportunities to target NosP pathways for practical applications of our discovery.
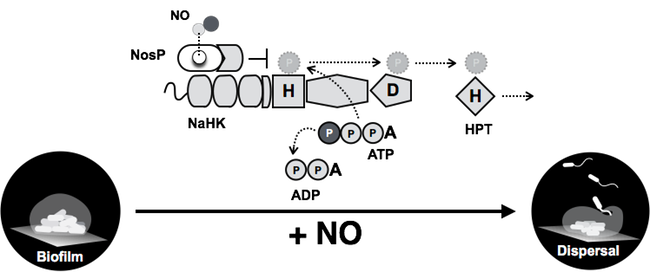
NosP is a NO sensor in P. aeruginosa that regulates the activity of hybrid kinase NahK. NahK regulates the phosphorylation state of the phosphotransfer protein HptB, which ultimately regulates a NO-mediated biofilm phenotype.
For more information see:
- Hossain,
S.; Heckler, I.; Boon, E.M.* (2018)
Discovery of a nitric oxide responsive quorum sensing circuit in Vibrio cholera. American Chemical Society Chemical Biology, 13, 1964–1969 (DOI: 10.1021/acschembio.8b00360).
- Williams, D.E.; Nisbett, L.M.; Bacon, B.A.; Boon, E.M.* (2017) Bacterial heme-based sensors of nitric oxide. Forum issue themed “Metallocofactors in Sensing,” Booker, S.J., ed. In Antioxidants and Redox Signaling, in press. (DOI: 10.1089/ars.2017.7235).
- Hossain, S.; Nisbett, L.M.; Boon, E.M.* (2017) Discovery of two bacterial nitric oxide-responsive proteins and their roles in bacterial biofilm regulation. Accounts of Chemical Research, 50, 1633-1639 (http://dx.doi.org/10.1021/acs.accounts.7b00095).
- Bacon,
B.A.; Nisbett, L.M.; Boon, E.M.*
(2017) Bacterial haemoprotein sensors of NO: H-NOX and NosP. Themed issue
“Metals and Antimicrobials,” Poole, R., ed. Advances
in Microbial Physiology, 70, 1-36 (DOI: 10.1016/bs.ampbs.2017.01.004).
- Hossain, S.; Boon, E.M.* (2017) NosP: Discovery of a nitric oxide binding protein and nitric oxide-responsive signaling pathway in Pseudomonas aeruginosa. American Chemical Society Infectious Diseases, 3, 454-461 (DOI: 10.1021/acsinfecdis.7b00027).
- Bacon, B.A.; Nisbett, L.M.; Boon, E.M.* (2017) Bacterial haemoprotein sensors of NO: H-NOX and NosP. In Advances in Microbial Physiology: Metals and Antimicrobials, 1-36. Poole, R., ed., Oxford, United Kingdom: Academic Press.
.