It is widely recognized that bis-(3’-5’)-cyclic-dimeric-guanosine monophosphate (cyclic-di-GMP; c-di-GMP) is a ubiquitous bacterial signaling molecule that balances motility and virulence in individual cells v. cell adhesion and persistence in biofilms. Although this is a relatively new area of inquiry, the emerging theme is that the total concentration of intracellular c-di-GMP is tightly regulated by a variety of enzymes that both synthesize and degrade c-di-GMP. C-di-GMP is synthesized by proteins with di-guanylate cyclase activity, identified by a GGDEF motif (a five amino acid conserved sequence) and often called a GGDEF domain. Proteins with phosphodiesterase activity degrade c-di-GMP to the linear dinucleotide pGpG. These are identified by a conserved EAL or HD-GYP amino acid motif and referred to as EAL or HD-GYP domains. The details of c-di-GMP regulation in bacteria are under investigation, but current evidence indicates that the activity of GGDEF, EAL, and HD-GYP domains are regulated by specific stimuli.
In the genomes of many bacteria, hnoX (heme-nitric oxide/oxygen binding) genes are located near genes coding for c-di-GMP synthases and/or phosphodiesterases. H-NOX proteins are evolutionarily conserved NO sensors. This genomic arrangement led us to hypothesize that NO-ligation to H-NOX could regulate biofilm formation through c-di-GMP signaling. Indeed, we have recently established that in some bacteria, NO/H-NOX reduces intracellular c-di-GMP concentrations through two mechanisms, ultimately dramatically inhibiting biofilm formation. In the absence of NO, H-NOX activates the c-di-GMP synthesis, but upon NO binding, H-NOX down-regulates di-guanylate cyclase activity and up-regulates phosphodiesterase activity.
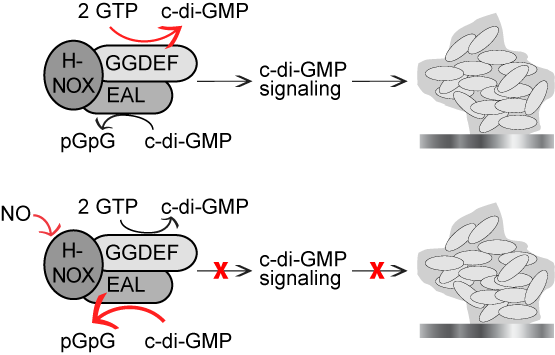
Schematic diagram of NO/H-NOX regulation of c-di-GMP concentrations through reciprocal regulation of c-di-GMP synthase and phosphodiesterase activities.
In our current and future work, we are expanding on this initial discovery in four directions: (i) delving deeper into the mechanism of NO/H-NOX regulation of c-di-GMP signaling; (ii) investigating this pathway as a target for anti-biofilm drug development; (iii) determining if this pathway is general and if it is involved in eukaryotic/bacterial interactions; and (iv) investigating alternate mechanisms of NO/H-NOX regulation of c-di-GMP concentrations.
For more information, see:
- Nisbett, L.M.; Boon, E.M.* (2016) Nitric oxide regulation of H-NOX signaling pathways in bacteria. Biochemistry, 55,4873-4884 (DOI: 10.1021/acs.biochem.6b00754).
- Arora, D.P.; Hossain, S.; Xu, Y.; Boon, E.M.* (2015) Nitric oxide regulation of bacterial biofilms. Biochemistry (DOI: 10.1021/bi501476n).
- Nesbitt, N.M.; Arora, D.P.; Johnson, R.A.; Boon, E.M.* (2015) Modification of a bi-functional diguanylate cyclase-phosphodiesterase to efficiently produce cyclic di-guanylate monophosphate. Biotechnology Reports (DOI: 10.1016/j.btre.2015.04.008).
- Lahiri, T.; Luan, B.; Raleigh, D.P.; Boon, E.M.* (2014) A structural basis for the regulation of an H-NOX-associated cyclic-di-GMP synthase/phosphodiesterase enzyme by NO-bound H-NOX. Biochemistry, in press (DOI: 10.1021/bi401597m).
- Arora, D.P.; Boon, E.M.* (2012) Nitric oxide regulated two-component signaling in Pseudoalteromonas atlantica. Biochemical and Biophysical Research Communications, 421, 521-526 (DOI: 10.1016/j.bbrc.2012.04.037).
- Liu, N.; Xu, Y.; Hossain, S.; Huang, N.; Coursolle, D.; Gralnick, J.; Boon, E.M.* (2012) Nitric oxide regulation of cyclic di-GMP synthesis and hydrolysis in Shewanella woodyi. Biochemistry, 51, 2087-2099 (DOI: 10.1021/bi201753f).
- Liu, N.; Pak, T.; Boon, E.M.* (2010) Characterization of a di-guanylate cyclase from Shewanella woodyi with cyclase and phosphodiesterase activities. Molecular BioSystems, 6, 1561-1564 (DOI: 10.1039/c002246b).