Quorum sensing regulates community-wide processes like biofilm formation, virulence gene production, and bioluminescence, in response to the detection of small molecules called autoinducers. Vibrio harveyi have three described quorum sensing circuits. Each involves the synthesis, secretion, and detection of a small molecule called an autoinducer. Autoinducer detection regulates phosphorylation of its cognate receptor kinase. Each receptor exchanges phosphate with a common phosphorelay protein, LuxU, which ultimately regulates bioluminescence through transcription factors named LuxO and LuxR (see figure).
We recently demonstrated that NO participates in quorum sensing through LuxU. In essence, NO enters quorum sensing circuits analogously to an autoinducer: it binds its cognate receptor (the H-NOX/HqsK complex; HqsK = H-NOX-associated quorum sensing kinase) and contributes to the flux of phosphate to and from LuxO. This discovery has set us up to investigate several important questions in our future work: (i) determining energetic and biophysical parameters of LuxU-based quorum sensing; (ii) assessing if NO/H-NOX quorum sensing occurs in other bacteria; and (iii) determining the NO source used in quorum sensing.
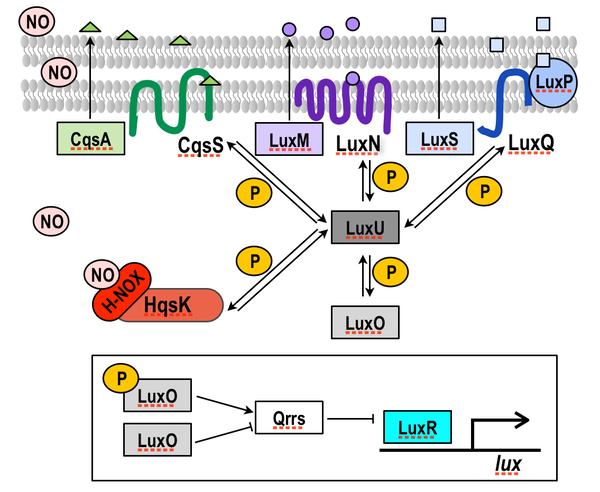
Schematic illustration of V. harveyi quorum sensing. P represents phosphate; green triangles, purple circles, and blue squares represent previously characterized autoinducers. Each autoinducer binds to and regulates phosphorylation of its cognate receptor (CqsS, LuxN, and LuxP/LuxQ). We have described a 4th quorum sensing pathway that is responsive to NO. NO/H-NOX regulates the phosphorylation state of HqsK, which along with the other autoinducer/receptor pairs, contributes to regulation of the phosphorylation state of LuxU, which ultimately regulates the quorum sensing response.
For more information see:
- Hossain,
S.; Heckler, I.; Boon, E.M.* (2018)
Discovery of a nitric oxide responsive quorum sensing circuit in Vibrio cholera. American Chemical Society Chemical Biology, 13, 1964–1969 (DOI: 10.1021/acschembio.8b00360).
- Nisbett, L.M.; Boon, E.M.* (2016) Nitric oxide regulation of H-NOX signaling pathways in bacteria. Biochemistry, 55,4873-4884 (DOI: 10.1021/acs.biochem.6b00754).
- Arora, D.P.; Hossain, S.; Xu, Y.; Boon, E.M.* (2015) Nitric oxide regulation of bacterial biofilms. Biochemistry (DOI: 10.1021/bi501476n).
- Henares, B.M.; Xu, Y.; Boon, E.M.* (2013) A nitric oxide-responsive quorum sensing circuit in Vibrio harveyi regulates flagella production and biofilm formation. The International Journal of Molecular Sciences, 14, 16473-16484 (DOI: 10.3390/ijms140816473).
- Henares, B.M.; Higgins, K.E.; Boon, E.M.* (2012) Discovery of a nitric oxide-responsive quorum sensing circuit in Vibrio harveyi. ACS Chemical Biology, 1331-1336 (DOI: 10.1021/cb300215t).
- Ueno, T.B.; Johnson, R.A.; Boon, E.M.* (2015) Optimized assay for the quantification of histidine kinase autophosphorylation. Biochemical and Biophysical Research Communications, in press (DOI: 10.1016/j.bbrc.2015.07.121).