Nitric oxide regulation of bacterial biofilms
Biofilms, surface-adhered bacterial communities, are widespread, persistent, and extremely resistant to antibiotics. Not surprisingly, they constitute a significant public health hazard; new strategies for biofilm prevention are desperately needed. Our group has taken the approach that the best way to eradicate biofilm infections is not to try to kill bacteria in the biofilm form, but to trick them into dispersing from biofilm, and thus restoring their susceptibility to antibiotics.
When we began our work, several reports had documented nitric oxide (NO) dispersion of biofilms through an unknown pathway. We observed that in many bacteria, hnoX genes neighbor genes encoding cyclic-di-guanidinemonophosphate (cyclic-di-GMP) processing enzymes and/or histidine kinases. We therefore hypothesized that two well-established biofilm regulatory mechanisms, intracellular cyclic-di-GMP signaling and intercellular quorum sensing, might be regulated by NO binding to hnoX-encoded proteins, i.e., H-NOXs. We have shown that H-NOX is a modular NO sensor that negatively affects biofilm formation, either:
(1) by directly regulating cyclic-di-GMP processing enzymes;
- Nesbitt, N.M.; Arora, D.P.; Johnson, R.A.; Boon, E.M.* (2015) Modification of a bi-functional diguanylate cyclase-phosphodiesterase to efficiently produce cyclic di-guanylate monophosphate. Biotechnology Reports, 7, 30-37 (DOI: 10.1016/j.btre.2015.04.008).
- Lahiri, T.; Luan, B.; Raleigh, D.P.; Boon, E.M.* (2014) A structural basis for the regulation of an H-NOX-associated cyclic-di-GMP synthase/phosphodiesterase enzyme by nitric oxide-bound H-NOX. Biochemistry, 53, 2126-2135 (DOI: 10.1021/bi401597m).
- Liu, N.; Xu, Y.; Hossain, S.; Huang, N.; Coursolle, D.; Gralnick, J.; Boon, E.M.* (2012) Nitric oxide regulation of cyclic di-GMP synthesis and hydrolysis in Shewanella woodyi. Biochemistry, 51, 2087-2099 (DOI: 10.1021/bi201753f).
- Liu, N.; Pak, T.; Boon, E.M.* (2010) Characterization of a diguanylate cyclase from Shewanella woodyi with cyclase and phosphodiesterase activities.Molecular BioSystems, 6, 1561-1564 (DOI:10.1039/c002246b).
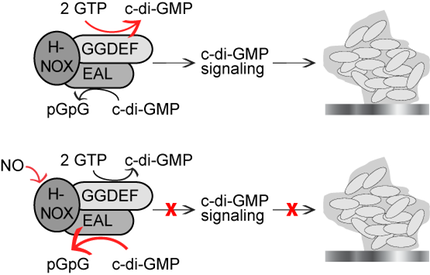
(2) by indirectly regulating cyclic-di-GMP processing enzymes through an intervening histidine kinase (we have also significantly contributed to the development of assays for assaying histidine kinases);
- Ueno, T.B.; Johnson, R.A.; Boon, E.M.* (2015) Optimized assay for the quantification of histidine kinase autophosphorylation. Biochemical and Biophysical Research Communications, 465, 331-337 (DOI: 10.1016/j.bbrc.2015.07.121).
- Arora, D.P.; Boon, E.M.* (2013) Unexpected biotinylation using ATP-γ-Biotin-LC-PEO-amine as a kinase substrate. Biochemical and Biophysical Research Communications, 432, 287-290 (DOI: 10.1016/j.bbrc.2013.01.115).
- Arora, D.P.; Boon, E.M.* (2012) Nitric oxide regulated two-component signaling in Pseudoalteromonas atlantica. Biochemical and Biophysical Research Communications, 421, 521-526 (DOI: 10.1016/j.bbrc.2012.04.037).
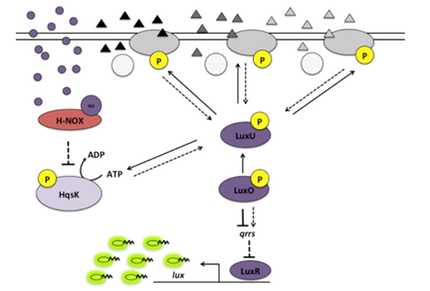
(3) or, sometimes, NO-bound H-NOX enters quorum sensing circuits through a histidine kinase.
- Ueno, T.; Fischer, J.T.; Boon, E.M.* (2019) Nitric oxide enters quorum sensing via the H-NOX signaling pathway in Vibrio parahaemolyticus. Fronteirs in Microbiology, 10:2108 (DOI: 10.3398/fmicb.2019.02108).
- Henares, B.M.; Xu, Y.; Boon, E.M.* (2013) A nitric oxide-responsive quorum sensing circuit in Vibrio harveyi regulates flagella production and biofilm formation. The International Journal of Molecular Sciences, 14, 16473-16484 (DOI: 10.3390/ijms140816473).
- Henares, B.M.; Higgins, K.E.; Boon, E.M.* (2012) Discovery of a nitric oxide-responsive quorum sensing circuit in Vibrio harveyi. American Chemical Society Chemical Biology, 7, 1331-1336 (DOI: 10.1021/cb300215t).
(4) We have also pursued understanding the molecular details of NO/H-NOX signaling.
NO binds H-NOX at the iron atom of a histidine-ligated heme cofactor that is severely distorted from planarity. We have demonstrated that heme flattening upon NO binding is the trigger for signal transduction.
- Sun, Y.; Benabbas, A.; Zeng, W.; Muralidharan, S.; Boon, E.M., Champion, P.M. (2016) Kinetic control of O2 reactivity in H-NOX domains. Physical Chemistry B, 120, 5351-5358 (DOI: 10.1021/acs.jpcb.6b03348).
- Kosowicz, J.G.; Boon, E.M.* (2013) Insights into the distal pocket of H-NOX using fluoride as a probe for H-bonding interactions. Journal of Inorganic Biochemistry, 126, 91-95 (DOI: 10.1016/j.jinorgbio.2013.05.012).
- Dai, Z.; Farquhar, E.R.; Arora, D.P.; Boon, E.M.* (2012) Is histidine dissociation a critical component of the NO/H-NOX signaling mechanism? Insights from X-ray absorbance spectroscopy. Dalton Transactions, 41, 7984-7993 (DOI: 10.1039/C2DT30147D).
- Muralidharan, S.; Boon, E.M.* (2012) Heme flattening is sufficient for signal transduction in the H-NOX family. Journal of the American Chemical Society, 134, 2044-2046 (DOI: 10.1021/ja211576b).
- Dai, Z.; Boon, E.M.* (2010) Engineering of the heme pocket of an H-NOX domain for direct cyanide detection and quantification. Journal of the American Chemical Society, 132, 11496-11503 (DOI: 10.1021/ja101674z).

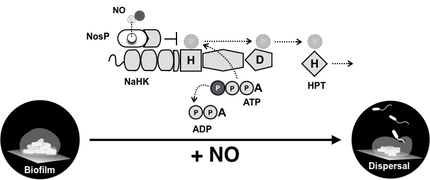
(5) Furthermore, we observed that NO regulates biofilm in species, including Pseudomonas aeruginosa, that lack hnoX homologs.
To bridge this knowledge gap, we discovered an additional heme-based NO sensor named NosP. Disruption of nosP in P. aeruginosa results in significantly less biofilm, and loss of the NO phenotype. Further, we have demonstrated that NO-bound NosP regulates downstream histidine kinases and cyclic-di-GMP synthesis/hydrolysis enzymes in many species.
- Nisbett, L.M.; Binnenkade, L.; Bacon, B.A.; Hossain, S.; Kotloski, N.J.; Brutinel, E.D.; Hartmann, R.; Drescher, K.; Arora, D.P.; Muralidharan, S.; Thormann, K.; Gralnick, J.A.; Boon, E.M.* (2019) NosP signaling controls the NO/H-NOX-mediated multicomponent cyclic-di-GMP network and biofilm formation in Shewanella oneidensis. Biochemistry, 58, 4827-4841 (DOI: 10.1021/acs.biochem.9b00706).
- Fischer, J.T.; Hossain, S.; Boon, E.M.* (2019) NosP modulates cyclic-di-GMP signaling in Legionella pneumophila. Biochemistry, 58, 4325-4334 (DOI: 10.1021/acs.biochem.9b00618).
- Bacon, B.; Liu, Y.; Kincaid, J.; Boon, E.M.* (2018) Spectral characterization of a novel NO-sensing protein in bacteria: NosP. Biochemistry (DOI: 10.1021/acs.biochem.8b00451).
- Hossain, S.; Heckler, I.; Boon, E.M.* (2018) Discovery of a nitric oxide responsive quorum sensing circuit in Vibrio cholera. American Chemical Society Chemical Biology, 13, 1964–1969 (DOI: 10.1021/acschembio.8b00360).
- Hossain, S.; Boon, E.M.* (2017) NosP: Discovery of a nitric oxide binding protein and nitric oxide-responsive signaling pathway in Pseudomonas aeruginosa. American Chemical Society Infectious Diseases, 3, 454-461 (DOI: 10.1021/acsinfecdis.7b00027).
In summary, we have discovered mechanisms underlying NO regulation of biofilms.
Our work is the starting point for deeper investigations into the role of NO in bacteria and bacterial/host interactions. These fundamental investigations will result in novel strategies for biofilm regulation with widespread application in medicine and industry.